Scientific Achievement
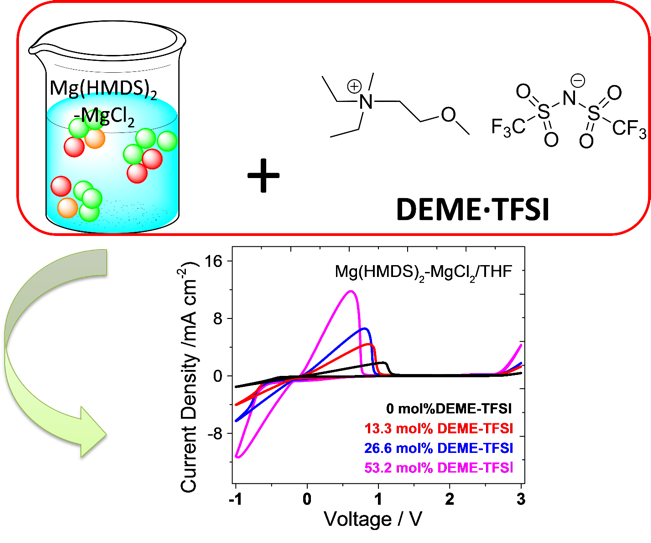
The effect of the addition of an ionic liquid DEME·TFSI to an electrolyte solution of Mg(HMDS)2-MgCl2 in THF was spectroscopically and electrochemically studied. Reversible magnesium deposition/dissolution and cycling of Mg-Mo6S8 coin cells were achieved with the DEME·TFSI-modified magnesium electrolyte.
Significance and Impact
Ionic liquids are thermally stable and non-volatile solvents, but they are largely incompatible with magnesium batteries. This study demonstrates the compatibility of the ionic liquid DEME·TFSI as an additive with magnesium deposition/dissolution and cycling of Mg-Mo6S8 coin cells, and provides insights to role of DEME·TFSI in the electrolyte.
Research Details
- Addition of DEME·TFSI into Mg(HMDS)2-MgCl2/THF electrolyte enables higher ionic conductivity of the electrolyte, which facilitates magnesium deposition/dissolution.
- Ionic conductivity measurements, cyclic voltammetry, SEM-EDS, NMR and Raman spectroscopy collectively suggest the role of DEME·TFSI in solution speciation of Mg(HMDS)2-MgCl2/THF electrolyte.

